How To Make Orbital Diagrams
Orbital diagrams — overview & examples Orbital electron orbitals atoms dimensional depicted Electron configuration orbital table periodic order chart config electrons configurations per shells following use just
Molecular Orbital Theory - Chemistry LibreTexts
Electron orbitals electronic chemistry quantum electrons numbers structure model atoms introductory orbital number figure atomic arrangement chem level energy libretexts Orbitals 3d representation chemistry chem libretexts et Drawing molecular orbital diagrams
Orbital electron diagrams configuration chemistry practice problems basic
Electron configuration orbital atomic electrons valence transition configurations metals phosphorus orbitals elements element chemistry diagrams level diagram state number atomOrbital molecular molecules diagram orbitals diatomic bonding of2 delocalized bond atomic libretexts electrons chem correlation hybridization atoms np homonuclear pageindex Electron configuration orbital diagram drawing diagrams configurations atoms aufbau arrangement rules exampleOrbital diagrams and electron configuration.
Orbital diagrams orbitals electrons overview monahanOrbitals molecular atomic molecules axis internuclear Orbital diagram scandium vanadium electron germanium configuration draw chemistry sc orbitals electronic 4p copper chromium exception 4s 4d answer studyLecture 7 presentation.

Molecular orbital theory
Orbitals atomic molecular orbital quantum number between difference px draw 2p 3d overlap principle 3dz atoms axes each coordinate ignoreDrawing atomic and molecular orbitals diagrams for molecules Electron configurations3.7: electron arrangement- the quantum model.
Drawing electron configurations with aufbau/orbital diagramElectronic configuration Electron configurations and atomic orbital diagramsOrbital electron diagrams configuration diagram potassium atom 2s configurations 1s 3s 2p ppt powerpoint presentation slideserve.

6.6: 3d representation of orbitals
Which are the orbitals(s,p,d,f) have center of symmetry?Orbitals shapes atomic draw 3d quantum subshell three many numbers shape configuration magnetic chemistry do orbital chemtube3d number level electrons Orbital orbitals subshell symmetry socraticOrbital molecular diagram cl2 s2 molecule bond orbitals electron unpaired bonding c2 energy theory valence electrons paramagnetic molecules diatomic atom.
Solved you can ignore the principle quantum number nOrbital molecular diagrams drawing Energy electron configuration orbital shell atomic levels level diagram filling chemistry periodic electronic iron atoms orbitals table electrons atom lowestOrbital diagrams.

How do you draw s,p,d,f orbitals?
Orbital elements diagrams notation which first lecture rules electronsChemical forums: molecular orbital diagram Electronic pairing structure orbital diagrams chemistry quantum diagram spin notation box electrons electron orbitals energy first boxes spins configurations levelCh150: chapter 2 – atoms and periodic table – chemistry.
.


Lecture 7 Presentation

Electron Configurations
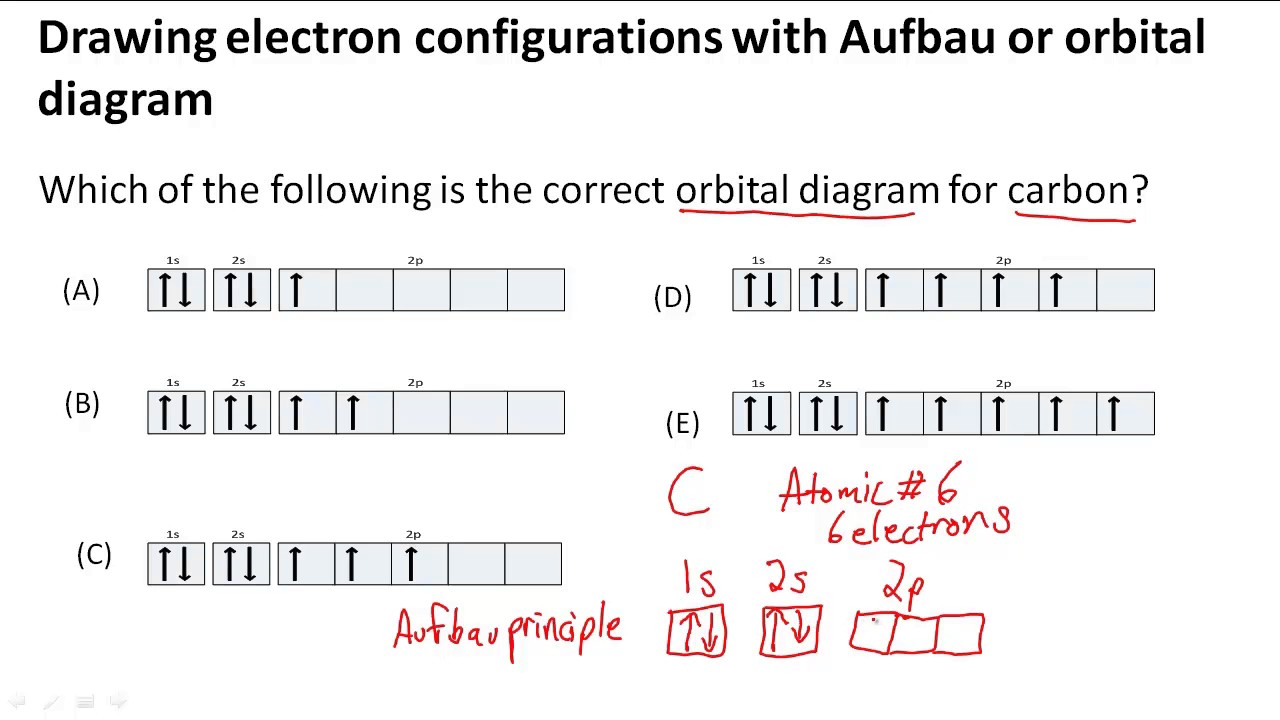
Drawing electron configurations with Aufbau/orbital diagram - YouTube

3.7: Electron Arrangement- The Quantum Model - Chemistry LibreTexts

Drawing Atomic and Molecular Orbitals Diagrams for Molecules - Organic

Solved you can ignore the principle quantum number n | Chegg.com

How do you draw s,p,d,f orbitals? | Socratic

Orbital Diagrams — Overview & Examples - Expii
